ModDetect™
Resource Hub
Whether you’re just discovering ModDetect or have already integrated it into your workflow, this hub brings together everything you need: application notes, protocols, publications, and support materials. From foundational guidance to in-depth insights, it’s your go-to source for trusted, up-to-date information.
Methods

Analysis of siRNA
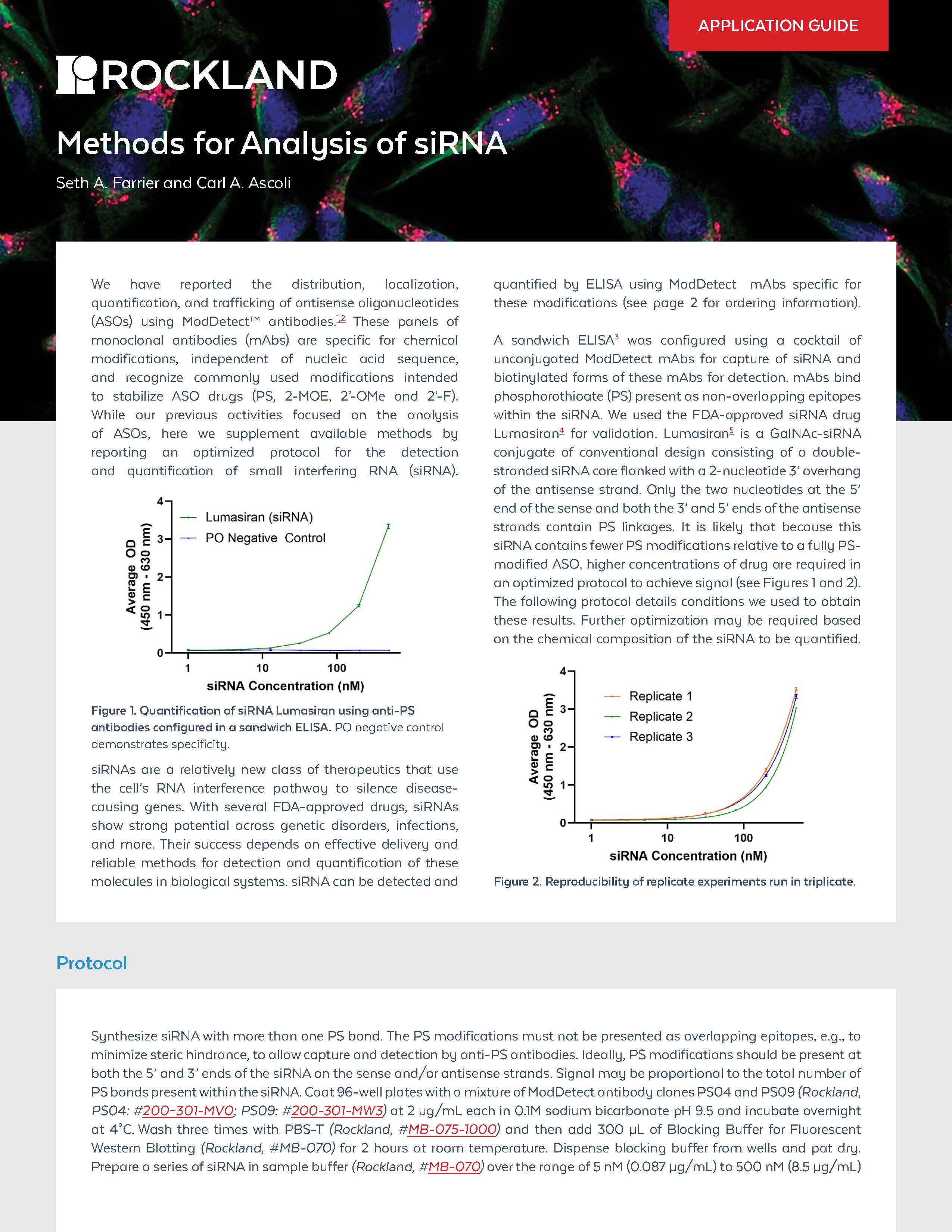
ModDetect™ antibodies enable sensitive detection and quantification of siRNA drugs such as Lumasiran, using sandwich ELISA formats specific for phosphorothioate bonds—offering a sequence-independent tool to track delivery, localization, and stability in therapeutic development


Analysis of Oligonucleotide Drugs
ModDetect™ panels provide universal, sequence-independent antibodies against backbone and sugar modifications, supporting ELISA, ICC, and IHC for ASO, siRNA, and mRNA, expanding beyond classical assays to improve sensitivity, visualization, and workflow efficiency in oligonucleotide drug research
Methods

Analysis of siRNA
ModDetect™ antibodies enable sensitive detection and quantification of siRNA drugs such as Lumasiran, using sandwich ELISA formats specific for phosphorothioate bonds—offering a sequence-independent tool to track delivery, localization, and stability in therapeutic development

Analysis of Oligonucleotide Drugs
ModDetect™ panels provide universal, sequence-independent antibodies against backbone and sugar modifications, supporting ELISA, ICC, and IHC for ASO, siRNA, and mRNA, expanding beyond classical assays to improve sensitivity, visualization, and workflow efficiency in oligonucleotide drug research
Posters
ModDetect™: A Universal Antibody-Based Approach for Oligonucleotide Detection
This introductory poster presents ModDetect, Rockland’s platform of validated monoclonal antibodies that recognize key chemical modifications in oligonucleotide therapeutics (ONTs), including phosphorothioate (PS), 2’-O-methoxy-ethyl (MOE), and 2’-O-methyl (OMe). Designed for use across diverse assay types, ModDetect provides an orthogonal alternative to LC-MS/MS and ligand binding assays by enabling direct localization and quantification of ONTs in lysates and biofluids. Explore how this powerful tool supports drug development pipelines by improving analytical confidence in ADMET and regulatory studies.
POSTERS
ModDetect™: A Universal Antibody-Based Approach for Oligonucleotide Detection
This introductory poster presents ModDetect, Rockland’s platform of validated monoclonal antibodies that recognize key chemical modifications in oligonucleotide therapeutics (ONTs), including phosphorothioate (PS), 2’-O-methoxy-ethyl (MOE), and 2’-O-methyl (OMe). Designed for use across diverse assay types, ModDetect provides an orthogonal alternative to LC-MS/MS and ligand binding assays by enabling direct localization and quantification of ONTs in lysates and biofluids. Explore how this powerful tool supports drug development pipelines by improving analytical confidence in ADMET and regulatory studies.
Immunoassays for ONTs: Localization, Quantification, and Trafficking Across Systems
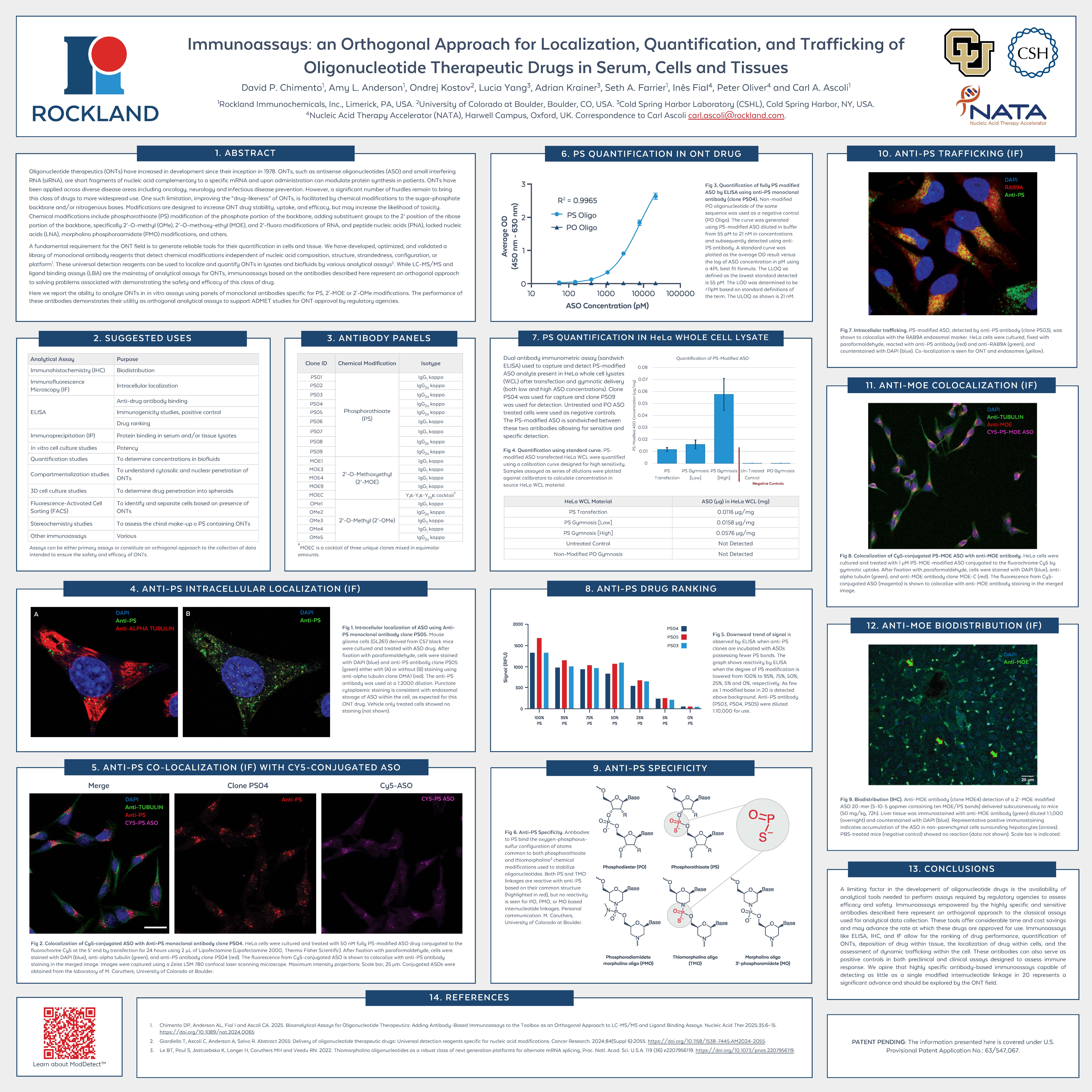
This poster presents a comprehensive application of ModDetect antibodies across a range of immunoassays, highlighting their versatility in localizing, quantifying, and tracking oligonucleotide therapeutics (ONTs) in serum, cells, and tissues. Using panels specific for PS, 2'-O-methyl (OMe), and 2'-O-methoxy-ethyl (MOE) modifications, the study demonstrates effective ONT detection in ELISA, immunofluorescence, and IHC formats. Key data include quantification from HeLa lysates, co-localization with Cy5-labeled ONTs and endosomal markers, and visualization of drug biodistribution in mouse liver tissue. This marks the first ModDetect poster to explore MOE-specific data in depth, underscoring its utility for preclinical and regulatory workflows through orthogonal detection strategies.
Immunoassays for ONTs: Localization, Quantification, and Trafficking Across Systems
This poster presents a comprehensive application of ModDetect antibodies across a range of immunoassays, highlighting their versatility in localizing, quantifying, and tracking oligonucleotide therapeutics (ONTs) in serum, cells, and tissues. Using panels specific for PS, 2'-O-methyl (OMe), and 2'-O-methoxy-ethyl (MOE) modifications, the study demonstrates effective ONT detection in ELISA, immunofluorescence, and IHC formats.
Key data include quantification from HeLa lysates, co-localization with Cy5-labeled ONTs and endosomal markers, and visualization of drug biodistribution in mouse liver tissue. This marks the first ModDetect poster to explore MOE-specific data in depth, underscoring its utility for preclinical and regulatory workflows through orthogonal detection strategies.
ModDetect in Action: Analytical Applications for ONT Development
After introducing the core capabilities of ModDetect, this poster showcases the next step: applying the platform in quantitative, regulatory-relevant workflows. Here, we present an ELISA-based assay optimized for detecting phosphorothioate (PS)-modified oligonucleotides in complex biological samples, including serum, blood, and tissue homogenates. This sensitive and high-throughput approach addresses key challenges outlined in FDA guidance—enabling robust ADMET and DMPK assessments critical to the clinical advancement of ONTs. With potential extensions into Biolayer Interferometry (BLI) and immunoprecipitation, this work positions ModDetect as a powerful tool for therapeutic monitoring and mechanistic insight.
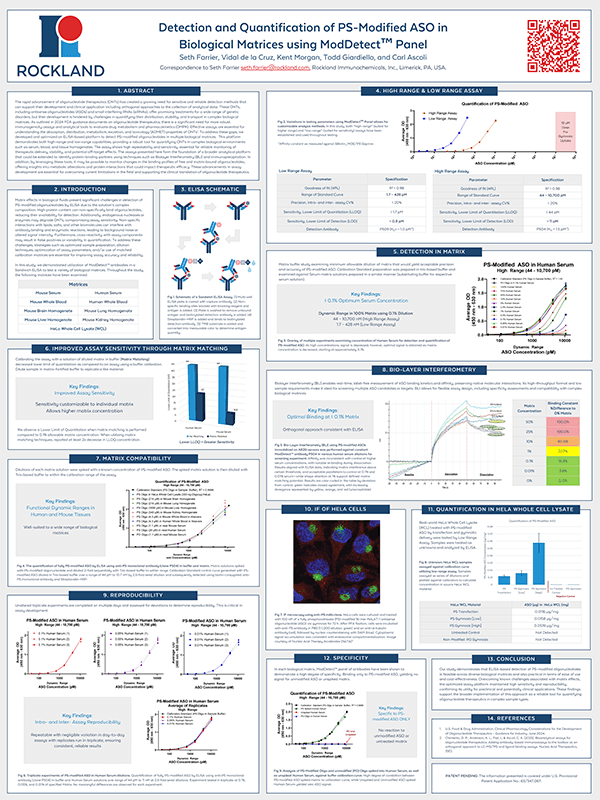
ModDetect in Action: Analytical Applications for ONT Development
After introducing the core capabilities of ModDetect, this poster showcases the next step: applying the platform in quantitative, regulatory-relevant workflows. Here, we present an ELISA-based assay optimized for detecting phosphorothioate (PS)-modified oligonucleotides in complex biological samples, including serum, blood, and tissue homogenates.
This sensitive and high-throughput approach addresses key challenges outlined in FDA guidance—enabling robust ADMET and DMPK assessments critical to the clinical advancement of ONTs. With potential extensions into Biolayer Interferometry (BLI) and immunoprecipitation, this work positions ModDetect as a powerful tool for therapeutic monitoring and mechanistic insight.
Manuscript
Manuscript
Bioanalytical Assays for Oligonucleotide Therapeutics
Since their discovery in 1978, nucleic acid therapies—including oligonucleotide therapeutics (ONTs) and vaccines—have grown rapidly, especially following the approval of the first mRNA COVID-19 vaccine in 2021. Now considered the third major drug class after small molecules and antibodies, ONTs like ASOs and siRNAs regulate gene expression by targeting RNA.
However, challenges such as instability, poor uptake, off-target effects, and immunogenicity hinder their development. Regulatory agencies, including the FDA, stress the need for improved analytical tools. We propose that immunoassays using highly specific anti-ONT antibodies offer a promising solution.
Protocols
Manuscript
Manuscript
Bioanalytical Assays for Oligonucleotide Therapeutics
Since their discovery in 1978, nucleic acid therapies—including oligonucleotide therapeutics (ONTs) and vaccines—have grown rapidly, especially following the approval of the first mRNA COVID-19 vaccine in 2021. Now considered the third major drug class after small molecules and antibodies, ONTs like ASOs and siRNAs regulate gene expression by targeting RNA.
However, challenges such as instability, poor uptake, off-target effects, and immunogenicity hinder their development. Regulatory agencies, including the FDA, stress the need for improved analytical tools. We propose that immunoassays using highly specific anti-ONT antibodies offer a promising solution.
Flyers
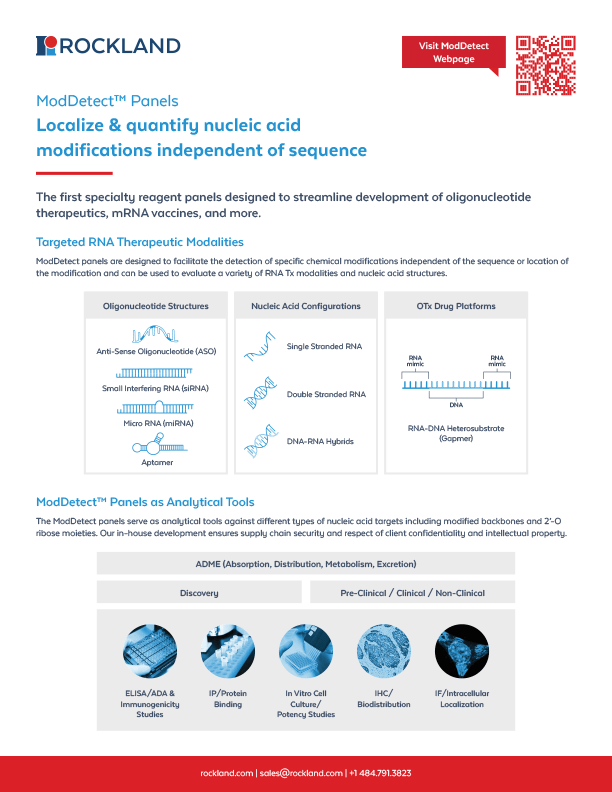
A fast, flexible reagent panel for
ONT detection
ModDetect™ Panels provide validated antibodies for identifying key chemical modifications in oligonucleotide therapeutics—supporting ELISA, IHC, IF, and more. Streamline your ONT workflow and reduce development time by up to 12 months.
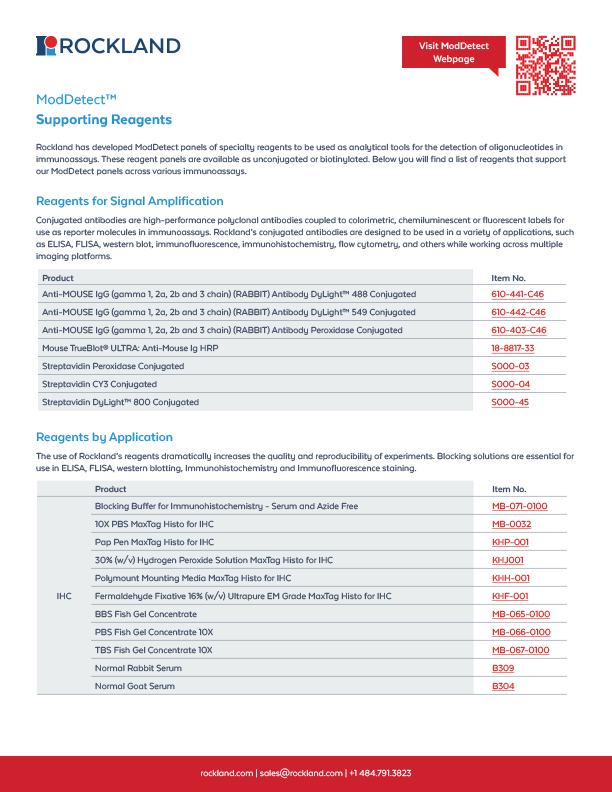
Supporting Reagents:
Tools to Power Your Assays
Explore our curated collection of buffers, conjugates, magnetic beads, and subcellular markers designed to optimize performance across ELISA, IHC, IF, and IP workflows. These reagents pair seamlessly with ModDetect™ panels to improve accuracy, signal clarity, and reproducibility in ONT detection and localization studies.
Publications
Interested in our ModDetect™ product line?
© 2025 Rockland Immunochemicals, Inc. All rights reserved.